

In this review article, we first describe an overview of diverse types of dendrite degeneration and regeneration in vertebrates and invertebrates. However, recent studies indicate that regulatory mechanisms of injury-induced dendrite degeneration and regeneration are distinct at least in part from mechanisms governing either developmental dendrite degeneration and regeneration or injury-induced axon degeneration and regeneration ( Stone et al., 2014 Thompson-Peer et al., 2016 Hao et al., 2019 Zhu et al., 2019).
The progression of injury-induced dendrite degeneration and regeneration are morphologically similar to what was observed in developmental dendrite degeneration and regeneration, respectively, suggesting that the developmental and injury-induced remodeling involve a shared program. In addition to developmental dendrite degeneration and regeneration, certain types of neurons remodel their dendritic arbors in response to injury on dendritic branches ( Richardson and Shen, 2019 Liu and Jan, 2020). These in vivo imaging studies have revealed that developing dendrites often undergo multiple rounds of regeneration and regeneration before the establishment of their final shape ( Yasunaga et al., 2010 Kaneko et al., 2011 Takeo et al., 2015 Nakazawa et al., 2018). Owing to technical advances in in vivo imaging, researchers are now able to fully trace branch dynamics of single neurons with high spatiotemporal resolution.

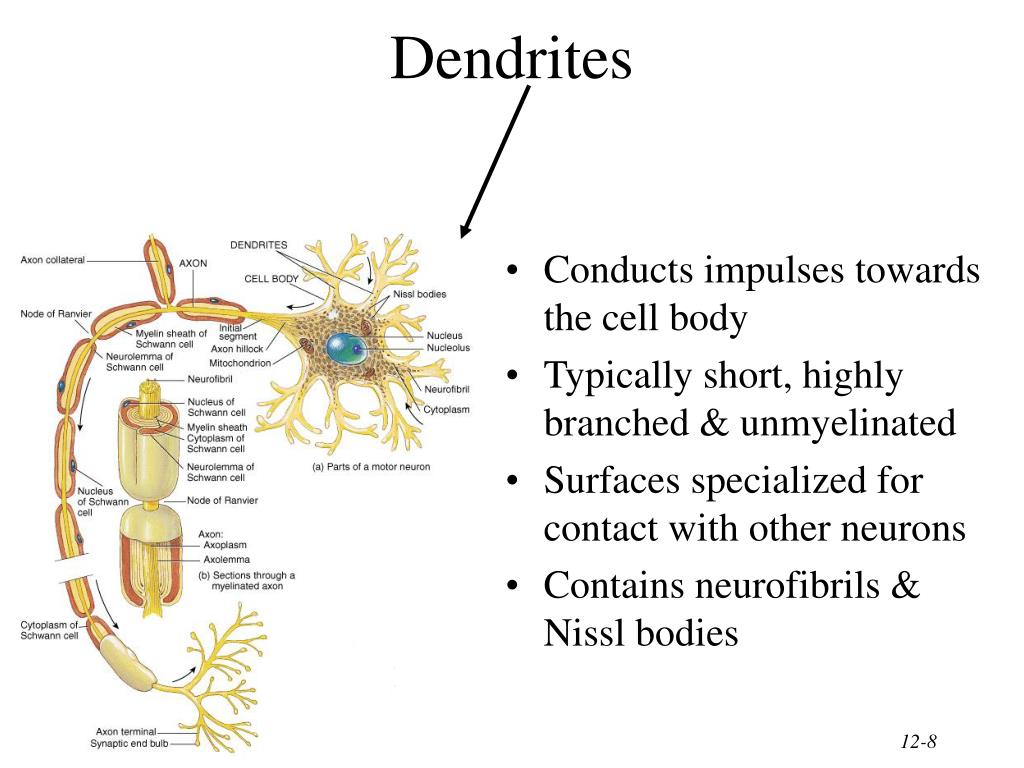
To achieve these changes in connectivity, neurons often remodel their dendrite shape through a combination of degeneration and regeneration of local dendritic branches ( Kanamori et al., 2015b Riccomagno and Kolodkin, 2015). During postnatal development of the mammalian brain, neurons exhibit extensive plasticity in which connectivity can be modified in response to neural inputs and/or hormonal regulation ( Parrish et al., 2007a Jan and Jan, 2010 Emoto, 2011 Batista and Hensch, 2019 Molnar et al., 2020). We further discuss how developmental dendrite degeneration and regeneration are molecularly and functionally related to dendrite remodeling in pathology, disease, and aging.ĭendrites are specialized structures designed to receive information from presynaptic neurons or sensory organs. In this article, we review recent progress in our understanding of dendrite degeneration and regeneration, focusing on molecular and cellular mechanisms underlying spatiotemporal control of dendrite remodeling in neural development. Dysregulation of these developmental processes, in particular dendrite degeneration, is associated with certain types of pathology, injury, and aging. In particular, dendrite degeneration must be targeted in a compartmentalized manner to avoid neuronal death. Both degeneration and regeneration of dendritic branches involve precise spatiotemporal regulation for the proper wiring of functional networks. Recent in vivo imaging studies reveal that the development of mature dendrite arbors in many cases involves extensive remodeling achieved through a precisely orchestrated interplay of growth, degeneration, and regeneration of dendritic branches. These elegant but complex structures are highly patterned across the nervous system but vary tremendously in their size and fine architecture, each designed to best serve specific computations within their networks.

2International Research Center for Neurointelligence (WPI-IRCN), The University of Tokyo, Tokyo, Japanĭendrites are cellular structures essential for the integration of neuronal information.1Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Tokyo, Japan.


 0 kommentar(er)
0 kommentar(er)
